
Watch
Ozone, the poisonous gas that allows there to be life

The International Day for the Preservation of the Ozone Layer is celebrated on September 16th. But how much do you know about this gas? Why is it so important for life on Earth? We asked Dr. Alcide di Sarra, an Italian scientist who works at the Laboratory of Observations and Measurements for the Environment and Climate of the Italian National Agency for New Technologies, Energy and Sustainable Economic Development (ENEA). He had just landed in Italy after a study and measurements campaign at the Thule Observatory in Greenland. This is what he told us.

Professor di Sarra, you have just returned from a mission in Greenland. Can you tell us what you were dealing with, what is your researsearch was concerned with?
Some of my colleagues and I returned to Greenland in August 2021, following a long period of time during which it was not possible to go due to the pandemic. I have been going to Greenland for more than 30 years to make measurements and do studies related to atmospheric processes. With some of my colleagues, we mounted the first instrument at the Thule High Arctic Atmospheric Observatory in 1990. That observatory began in the early 90s, and it is where we carry out research together with La Sapienza University in Rome, ENEA, the National Institute of Geophysics and Volcanology (INGV), and the University of Florence. The observatory was created to study the processes related to the destruction of ozone in the Arctic because, in those years, it was one of the central research topics in atmospheric physics. We continue to study those processes now, but we are focusing mainly on phenomena related to climate-related variations. Because the Arctic is the region of the Earth that is warming faster than the rest of the planet. There are many complex processes of interconnection between the atmosphere, ocean, ice, atmospheric circulation, marine circulation that cause a very, very, strong warming to occur in this region, which goes under the name of Arctic amplification, precisely because the temperature in the air is rising about three times faster than the rest of the planet.

I see. So, his research also focuses on the observation of the famous ozone hole (for us who grew up in the 1980s). But, can you explain us, in simple terms, what it is?
So, the ozone hole is a seasonal phenomenon, discovered in 1985 by some British researchers while taking measurements from an Antarctica station called Halley Bay, with an instrument that measures the ozone content in a column of air. By making these measurements, which use solar radiation – at the onset of solar radiation, that is, at the beginning of the Antarctic spring – the researchers realized that there had been a very, very strong decrease in ozone from the end of the 1970s to 1985.
This reduction, which then progressed significantly over Antarctica, destroyed in the months of September, October and November up to about 65-70% of the ozone that was present above the column, involving a very extensive are; that is, the whole Antarctic region, which is a larger area than North America. There are about 25 million square kilometers in which the ozone concentration on the column is very low.
The “ozone hole” is therefore a seasonal phenomenon. It starts at the end of the Antarctic winter and in August. It deepens in September and the maximum destruction of ozone is reached in November-December. The phenomenon stops, the air mixes with air masses coming from other regions, and the so-called hole closes.
Something similar but on a much smaller scale also occurs in the Arctic – with much greater variability, because Arctic conditions are more unstable than Antarctic ones. Therefore, in just a few years, in spring, this destruction of the ozone is activated above the Arctic, which can reach 35-40%. But it is a much more intermittent situation than the regularity of the Antarctic phenomenon.
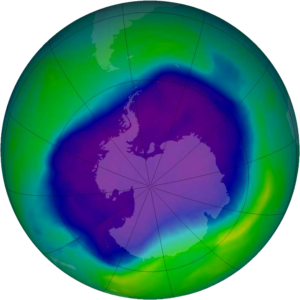
If I understand correctly, alongside this ozone destruction that produces holes, there is also a global process of ozone depletion in the stratosphere, which is not limited to areas above Antarctica or the Arctic.
The greatest concentration of ozone is found at an altitude of around 25km. In the stratosphere therefore, the region of the atmosphere from 10-15 km high to 50 km high. The processes that occur in the Antarctic winter, when temperatures in the stratosphere become very low, favor the formation of clouds, despite the very low concentration of water vapor and other chemical compounds. On the surface of these clouds, when solar radiation arrives, a series of chemical reactions occur that cyclically destroy the ozone. Destruction occurs in the stratosphere precisely because clouds form the stratosphere. When this low ozone concentration zone mixes with the rest, it contributes to impacts on the ozone layer in the stratosphere on a global scale, so a very strong ozone depletion was seen in the early 1990s. A very significant decrease which then slowed. So we now find ourselves, globally, with an ozone content that is around 3-4% lower than what it was in the early 1980s. In the last 15-20 years, the situation has become chronic, very regular, with a very strong decrease. However, the area hit by the ozone hole has stopped growing.
But can’t this situation be reversed somehow?
Yes, yes. There is a way, and it is also one of the greatest examples of international initiatives that have worked. I’m talking about the Montreal Protocol. Let me explain. One of the important compounds involved in these reactions is the chlorine atom. The compounds that contain it are mainly produced by anthropogenic activities. Some of the chemical compounds we produce that contain chlorine have a strong stability in the lower atmosphere, and they slowly reach the upper atmosphere. There they are dissociated from short wavelength solar radiation, the more energetic one, and thus can activate these destructive reactions.
If we facilitate a reduction in the chlorine concentration, this process slows down. And the further failure of the ozone hole to grow is mainly due to the fact that international agreements have limited emissions of chlorine compounds. So, the concentration of chlorine in the stratosphere began to decline. The effects are beginning to be seen, but these processes take a long time. Because these compounds have a very long life span: tens, in some cases, hundreds of years. Therefore, the concentration of chlorine in the stratosphere falls very slowly, because we still have the effects of substances that were put into the atmosphere many years ago. However, there are positive signs. And this is a great and successful example of an international agreement. Because the ozone hole was discovered in 1985 and the Montreal Protocol is from 1987. So, international initiatives were important, and they were done on time, very quickly.
Can you explain what the agreement says?
The Montreal Protocol – although there have been subsequent agreements and amendments – reduced emissions and the production of compounds that contain chlorine and, with regard to some gases, it has even eliminated them. In the beginning, some substitutes were included. From CFCs (chlorofluorocarbons) we moved on to HCFCs (hydrochlorofluorocarbons), then to HFCs (hydrofluorocarbons). The compounds used have a slightly shorter life span and, flourine atoms instead of chlorine atoms. They pose other problems but let’s say that this favors the reduction of the chlorine concentration in the stratosphere which has led to a certain recovery of the ozone content in the stratosphere on a global scale.Therefore, there are positive signs of an international initiative that has been ratified by all the nations of the world.
UNEP says that “all life on Earth depends on the existence of a thin screen of a poisonous gas high up in the atmosphere: the ozone layer.” Why is ozone so important for life on Earth?
The ability to absorb ultraviolet radiation, which is the most energetic form that comes from the sun, is also the main, or one of the major beneficial factors of ozone in the stratosphere. Because, by absorbing the most energetic radiation, which is the one that produces damage, it allows the surface of the earth to be livable. It is a very important factor for the life of human beings, but also of plants, for matter, and for what is found on the surface of the Earth. Various processes, from cataracts to skin cancers, depend on the amount of ultraviolet radiation that is shielded by the ozone.

Does this depletion of ozone also affect climate change?
There are links and there are many. For example, ozone has the ability to absorb infrared radiation and therefore, like the gases that absorb radiation in this spectral region, it is a greenhouse gas. So, in some ways, it is related to processes that also affect climate change. But there are several other important links that link ozone to the climate. For example, the gases that lead to the destruction of ozone are gases that also have a strong ability to absorb infrared radiation, so they are important greenhouse gases. Therefore, limiting these gases also means reducing the impact on the climate. One of the agreements following the Montreal Protocol, the Kigali Agreement, limited HFC (hydrofluorocarbon) emissions which, if not regulated, would have led to an additional temperature increase of a few tenths of a degree. Hence, they would have contributed significantly to global warming.
Another strong link between ozone and climate is linked to the anthropogenic greenhouse effect. One of the effects we know of, regarding the increase of greenhouse gases in the atmosphere, is low-altitude warming with simultaneous cooling in the stratosphere. This is one of the distinctive features that allow us to say that the temperature variation we see is due to the increase in greenhouse gases. We see warming below, and cooling in the stratosphere. This cooling in the stratosphere is one of the processes that slows down the recovery of the ozone hole, because if the temperature in the stratosphere decreases, it is easier for clouds to form and therefore all the processes that destroy ozone that occur on the surface of the clouds in the polar areas.
So, let’s say that there are multiple connections, the bonds are strong and the processes are strongly linked. Therefore, you can’t look at only one aspect and leave out all the rest. We must always consider the system in its complexity, in its complicated interactions and in the possible interference of one process with respect to the other.
Is there anything that can still be done to improve the ozone situation and consequently the climate?
There is a lot to do. There are still a variety of mechanisms that are still to be understood. For example, not long ago, thanks to the observation of the concentration of CFC in the atmosphere, it was discovered that there were still hidden sources that were thought to have been eliminated. Now we are worrying about all the CFCs contained in old refrigeration systems that have been decommissioned, which could bescattered in the atmosphere, producing some impact … In my opinion, it is important to continue to pay attention to this issue and continue to study it. Follow the evolution, don’t stop taking measurements, understand what the processes are, what impact human activity produces.

And what about our own actions in everyday life?
A few years ago, for example, one could choose a refrigerator or a car air-conditioner, epending on the compounds they contained. Nowadays most of those compounds no longer contain chlorine, so some choices have been forced on us by international agreements. I believe that one of the things we need to do now is to keep in mind that we live within an ecosystem that is extremely complex and interconnected and therefore to bear in mind that the choices we make, the initiatives we take, have consequences.
Therefore, maintain this attitude of understanding that we are in a complex system, to which there are no simple solutions or simple answers. But each of our actions has its impact and therefore it is important to have a mental approach that leaves us open to say: Let’s try to better understand how things are. Never be satisfied with a simple description of the phenomena. I think this is important to be able to balance our everyday choices. Not a very practical answer, though …
… but it conveys the point and urges us not to be satisfied with a simple web search to know more.
Exactly! Find reliable sources of information.



